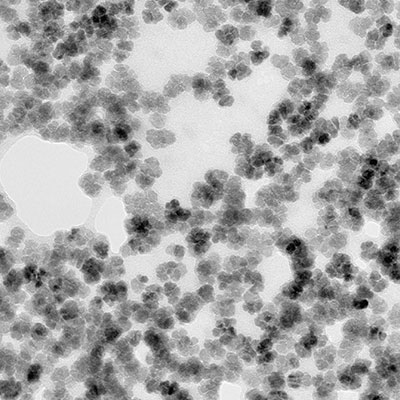
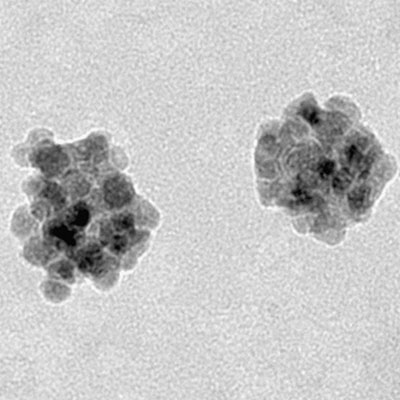
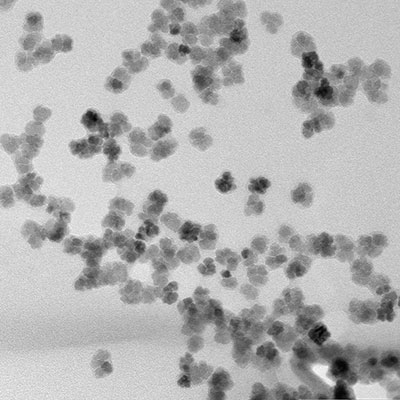
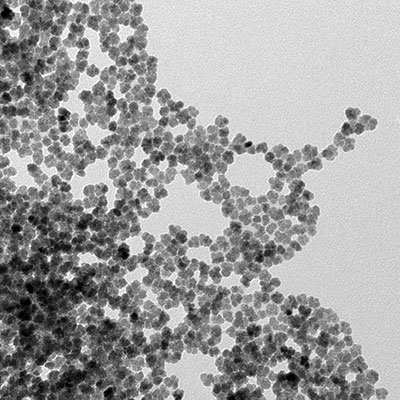
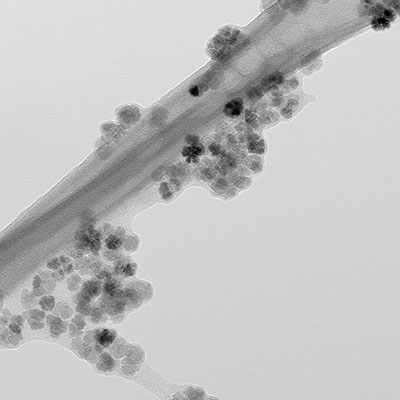
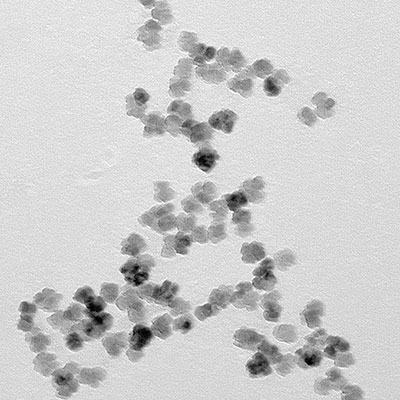Therapy
Our nanoparticles find important applications in antitumor therapies since they are valid means by which magnetic or laser hyperthermia and drug delivery can be performed:
- MAGNETIC FLUID HYPERTHERMIA
- LASER HYPERTHERMIA
- DRUG-DELIVERY
MAGNETIC FLUID HYPERTHERMIA
Hyperthermia therapy, a form of cancer treatment with elevated temperature in the range of 41–45°C, has been recently received considerable attention because it is expected to significantly reduce clinical side effects compared to chemotherapy and radiotherapy and can be effectively used for killing localized or deeply seated cancer tumours. Accordingly, various forms of hyperthermia have been developed in the past few decades to provide cancer clinics with more effective and advanced therapy techniques. Clinical feasibility of treating cancer by hyperthermia alone and along with other modalities –Radiation and Chemotherapy– has been previously investigated, but most of the proposed methods have limitations. These are due to the inability to reach optimal temperature especially in deep-sited tumour, to the invasive thermometry, and to lacking of differentiation among healthy and tumour cells.
Current developments in nanotechnology have provided a new set of systems, methodologies and materials for biomedical research and applications. These developments in biomedical research and nanotechnology accelerated a new form of hyperthermia treatment, the so-called magnetic nanoparticles hyperthermia, which relies on the insertion of nanosized (< 100 nm) heating mediators within the tumour site. These mediators are expected to resonantly respond to a time-varying magnetic field in the 50-500 kHz range, thus converting the electromagnetic energy into heat.
This new form of hyperthermia treatment is expected to provide a great deal of technical advantages:
- – direct injection of hyperthermia agents as particles dispersion through blood vessel;
- – easy transport of nanoparticles to the target cell by externally controlled magnetic field, or possibility for differentiation of tumour cells from healthy cells by using antibody-antigen biological reaction;
- – small heating loss during hyperthermia due to direct heating of cell;
- – selective heating of damaged tissues by the employment of electromagnetic field frequencies at which tissues not containing the magnetic mediators are nearly transparent.
A great advantage of using nanoparticles is their high effective surface area (easier attachment of ligands), lower sedimentation rates (high stability) and improved tissular diffusion. Moreover, the control and tailoring of the nanoparticles physical properties opens the possibility to use these systems, also simultaneously, for many other biomedical applications, such as contrast agent for magnetic resonance imaging – MRI and in-situ drug release.
LASER HYPERTHERMIA
Laser irradiation at near-infrared (NIR) frequencies can penetrate tissues with sufficient intensity and higher spatial precision for inducing localized hyperthermia, but effective mechanisms are needed to transduce light energy into heat and to discriminate unhealthy cells from healthy ones.
One promising approach is to introduce photothermal agents in the form of anisotropic gold nanoparticles, which can support plasmon resonances with remarkably high absorption cross sections at NIR wavelengths. Plasmon-resonant gold nanoparticles are appealing in several respects: (i) their intrinsic toxicity is low; (ii) their optical resonances can be optimized for specific NIR frequencies as a function of particle size and shape; (iii) their small sizes (<100 nm) are amenable to extravasation and adsorption by diseased cells and tissues; and (iv) their plasmon-enhanced properties enable them to serve as contrast agents for biological imaging. Coincidentally, it has been shown that hyperthermia can promote the uptake of nanoparticles by increasing tissue permeability and blood vessel dilation, suggesting a potential for synergistic effects. The development of plasmon-resonant nanoparticles as multimodal agents for biomedical diagnostics and therapeutics has become an area of intense research activity, with reports from several groups using gold nanospheres, nanoshells, and most recently nanorods.
Gold nanorods are especially attractive for applications of this sort: they can support a higher absorption cross section at NIR frequencies per unit volume than most other types of nanoparticles, and are readily prepared in micellar surfactant solutions using seeded growth conditions. Treatment of nanorods provides fine control over their longitudinal plasmon resonances and optical stability over time, facilitating their practical application. Nanorods also exhibit significantly narrower linewidths than spherical nanoparticles at comparable resonance frequencies due to reduced radiation damping effects, and have shown to be highly efficient at converting light energy into heat, with the highest thermal gradients produced by nanorods embedded in media having low thermal conductivity.
DRUG-DELIVERY
Nanoparticles represent drug delivery systems suitable for most administration routes. Over the years, a variety of natural and synthetic polymers have been explored for the preparation of nanoparticles, of which Poly(lactic acid) (PLA), Poly(glycolic acid) (PGA), and their copolymers (PLGA) have been extensively investigated because of their biocompatibility and biodegradability. Nanoparticles act as potential carriers for several classes of drugs such as anticancer agents, antihypertensive agents, immunomodulators, and hormones; and macromolecules such as nucleic acids, proteins, peptides, and antibodies. The options available for preparation have increased with advances in traditional methods, and many novel techniques for preparation of drug-loaded nanoparticles are being developed and refined. Nanoparticles can be designed for the site-specific delivery of drugs. The targeting capability of nanoparticles is influenced by particle size, surface charge, surface modification, and hydrophobicity. Finally, the in vivo performance of nanoparticles is influenced by morphological characteristics, surface chemistry, and molecular weight. Careful design of these delivery systems with respect to target and route of administration may solve some of the problems faced by new classes of active molecules.
The development of nano-encapsulation techniques nowadays provide a powerful tool for the controlled drug delivery, with imaging capabilities for monitoring the efficacy of the localised action.
The results achieved in current drug delivery techniques will lead to a change in the current technology, leading to novel nanocapsules as well as an update/optimisation of the existing manufacturing processes. The development of “target” application of nanocapsules for “specific disease” represents a relevant opportunity for young entrepreneurs and spin-off actions supported by national authorities. The new drug delivery techniques will open the doors to use multi-modal nanomaterials for other types of cancer as well as gain the trust of clinicians to adopt new therapy strategies that use magnetic hyperthermia.